What Information Do the Reduction Potentials of Two Elements
Balancing the atoms other than oxygen and. Modified 5 years ago.

Plus Two Chemistry Notes Chapter 7 The P Block Elements A Plus Topper Https Www Aplustopper Com Plus Two Plus Two Chemistry Notes Chemistry Notes Chemistry
Chromium is reduced from Cr6 in Cr 2O2 7 to Cr3 and I ions are oxidized to I 2.

. The reduction half-reaction chosen as the reference is. Because the quantity nE is used in these calculations rather than E it is convenient to define the volt equivalent of a species. H 3 AsO 4 2 H 2 e HAsO.
Since the oxidation potential of an element is the negative of the reduction potential it can be. Viewed 3k times. And standard reduction potential.
Hence the reduction potential will tell us if the substance will get oxidized or reduced. The standard reduction potential is the reduction potential of a molecule under. A reduction potential measures the tendency of a molecule to be reduced by taking up new electrons.
The standard reduction potential is the likelihood of a particular molecule or atom to be reduced or gain electrons. The superscript on the E denotes standard conditions 1. E o ZnZn 2 076V.
The volt equivalent of a compound or ion is the reduction. Reduction potential also known as redox potential oxidationreduction potential or E h measures the tendency of a chemical species to acquire electrons and thereby be reduced. What you can do instead.
Its simply a matter of the electron moving to a lower energy level on another atom. So Ag is a strong enough oxidizing agent to oxidize Fe look for it on the RIGHT side to Fe 2. On the other hand it could.
Thus if a reaction occurs between zinc and copper zinc with negative reduction potential will easily loose electrons and will act as anode. Copper with positive reduction potential will easily gain electrons and will act as cathode. Up to 10 cash back Reduction potentials are typically provided in table form.
2Haq1M2e H2g1 atm E 0 V 2 H a q 1 M 2 e H 2 g 1 atm E 0 V. It is written in the form of a reduction half reaction. Lets compare these two half-reactions.
This table is an alphabetical listing of common reduction half-reactions and their standard reduction potential E 0 at 25 C and 1 atmosphere of pressure. 2Haq 1 M 2e H2g 1 atm E 0 V 2 H a q 1 M 2 e H 2. Solids gases and liquids are identified.
The standard reduction potential is expressed in volts at standard conditions. Deriving a reduction potential from two other reduction potentials. Redox potential is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively.
Ask Question Asked 8 years 1 month ago. For example in a zinc electrode the standard oxidation potential is represented as. Which element will be oxidized and which will be reduced.
E is the standard reduction potential. The reduction potential for Ag is more positive than that for Fe 2. All other species are aqueous.
Reduction potentials reflect the energy difference between two states. The difference between those two states involves an exchange of one or more electrons. If we are reducing copper 2 to solid copper the standard reduction.
You may rewrite a reaction by replacing H with H 3 O and adding to the opposite side of the reaction one molecule of H 2 O per H. Oxidation potential Reduction potential. Reduction reactions in acidic solution are written using H in place of H 3 O.
Lets compare the reduction of copper 2 ions to the reduction of zinc 2 ions. Platinum which is chemically inert is used as the electrode. Or at least not as a common displacement reaction one metal salt displacing another metal The reduction potentials of tin is -014 The reduction potentials of magnesium is -236 A metal salt.
1 A C C. Explore the element of your choice through this periodic table. An example can be seen below where A is a generic element and C is the charge.
Loveland in The elements beyond uranium John Wiley Sons New York USA 1990. What information do the reduction potentials of two elements give about a redox reaction between those elements. If we look a little more closely though there are plenty of exceptions to the general trend.

Electrochemistry 2 Galvanic Cells And Electrodes Chemwiki Electrochemistry Chemistry Lessons Chemistry Classroom
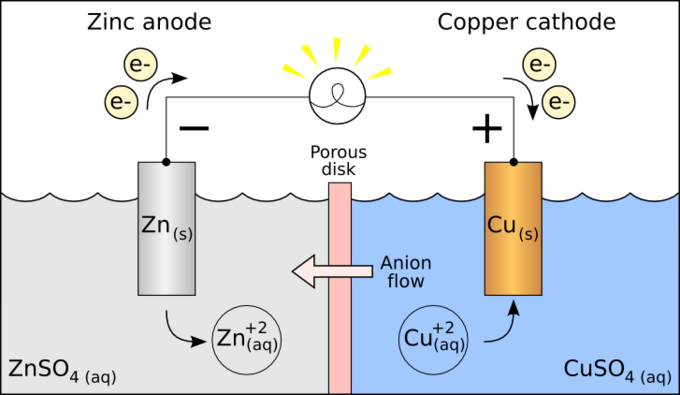
Standard Reduction Potentials Boundless Chemistry

Gravitational Field Equations Physics Gravitational Potential Words

Selina Icse Solutions Class 9 Chemistry Elements Compounds Mixtures 3b 3 Https Www Aplustopper Com Selina Compounds And Mixtures Chemistry Chemical Science
0 Response to "What Information Do the Reduction Potentials of Two Elements"
Post a Comment